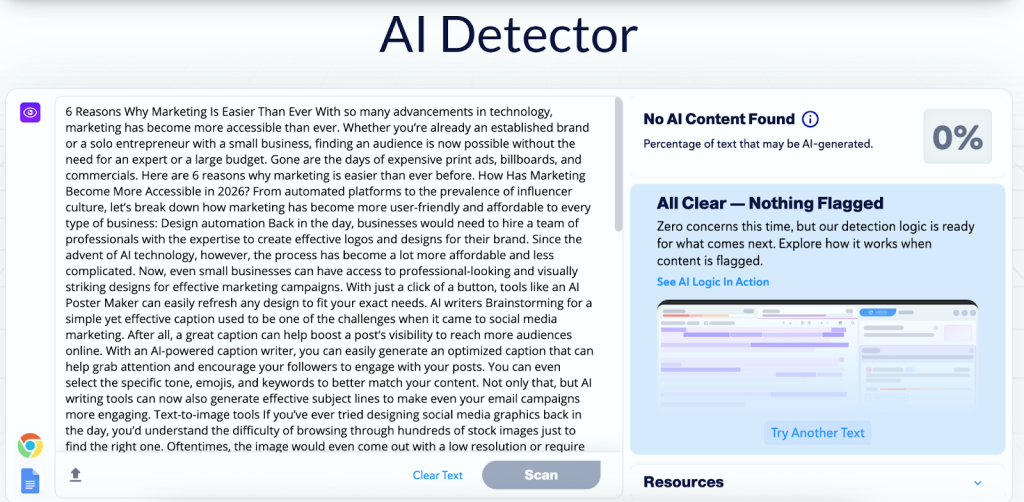
An automated exterior defibrillator (AED) [Photo by James Rein]
The Fda has extra automatic exterior defibrillators (AEDs), upper body drains/suction canisters and autotransfusion units to its record of health care equipment in limited offer.
AEDs — like wearable and nonwearable versions of the gadgets — are envisioned to be in confined supply for at least the relaxation of 2022, the Fda explained. The agency cited both of those an boost in demand for AEDs and the world-wide shortage of semiconductors applied in the units.
“The Food and drug administration carries on to work with federal companions and other stakeholders to assistance mitigate issues involved with semiconductor shortages,” the Food and drug administration explained in yesterday’s update to the system shortage checklist. “In addition, the Fda has issued direction documents, like enforcement guidelines, with regards to situations the place manufacturers may well look at modifying their products mainly because of source chain difficulties, these types of as semiconductor source difficulties.”
The Food and drug administration stated enhanced demand is also guiding the scarcity of upper body drains/suction canisters and autotransfusion techniques, nevertheless it could not estimate how long the scarcity would past. The Fda did not discover the semiconductor lack or other component, section or accessory supply problems for these gadgets.
At the exact same time, the regulatory company removed professional medical gowns and surgical masks from its health-related machine shortage listing.
Under the Coronavirus Aid, Relief, and Economic Security Act (CARES Act), medical gadget producers are expected to notify the Fda of production interruptions for crucial products and solutions if they’re very likely to lead to significant supply disruptions in the U.S.
Semiconductors have been in quick provide owing to all over the COVID-19 pandemic, largely due to fabrication disruption (which include fabs in Texas knocked offline by a cold-weather electricity outage final year) and large desire for autos and purchaser electronics.
The clinical device field only helps make up about 1% of the international desire for semiconductors, but even giants like Medtronic have had a really hard time obtaining sufficient chips — or particularly the right variety — for their devices.
The clinical product industry’s Sophisticated Health-related Know-how Association (AdvaMed) has lobbied the Biden administration to prioritize chip supplies for the medical system field, while lawmakers are advancing a $52 billion offer of subsidies for domestic chip manufacturing.
Similar: 5 ways to support healthcare product makers deal with semiconductor shortages