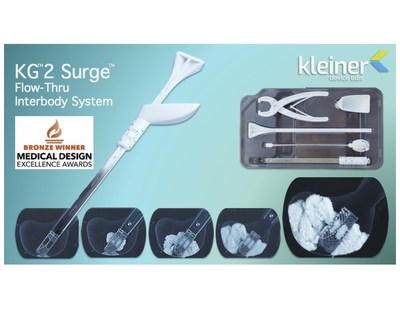
INCLINE VILLAGE, Nev., April 13, 2022 /PRNewswire/ — Kleiner Device Labs today announced that its new KG2™ Surge™ flow-thru interbody system was recognized with the Bronze award in the 2022 Medical Design Excellence Awards (MDEA) in the Implant and Tissue-Replacement Products category.
The KG2 system maximizes total bone graft delivery volume and streamlines TLIF and PLIF spinal fusion procedures.
The KG2 system maximizes total bone graft delivery volume, better distributes graft bilaterally into the intervertebral disc space, and streamlines the implant delivery, positioning and grafting processes for TLIF and PLIF spinal fusion procedures.
The annual MDEA competition recognizes products that are moving the $450 billion medical device industry forward through live-saving innovations and remarkable technological advancements. The MDEAs are determined each year by an independent panel–clinicians, engineers and designers—to select finalists as well as winners in each of 10 product categories.
“We are proud to earn this recognition of the many years of work and innovation that have gone into developing the company’s unique flow-thru technology that is key to the new KG2 interbody system and our future product pipeline,” said Jeff Kleiner, MD, founder and CEO of Kleiner Device Labs.
The FDA-cleared KG2 Surge system streamlines the implant delivery and grafting steps in lumbar spinal fusion procedures. The system comes with the interbody device pre-assembled with the cannula in a single-use sterile tray. It allows the surgeon to distract the disc space by inserting the implant, and then pack the disc space bilaterally through the KG2 Surge implant, using the attached cannulated inserter. The implant’s I-beam architecture and ramp design, combined with the large cross-section cannula, allows simplified delivery and improved fill volume of a wide range of allograft and autograft products, including viscous and fibrous materials.
For KG2 videos, technical specifications, instructions for use, or clinical efficacy information, please go to the company’s web site.
About Kleiner Device Labs
Kleiner Device Labs is creating new instruments and devices to advance minimally invasive spine surgery and improve outcomes and costs. Kleiner Device Labs is headquartered in Incline Village, Nevada.
KG and Surge are trademarks of Kleiner Device Labs.
Sales and Surgeon Contact:
Mike Hughes
[email protected]
M: 949.632.0769
Media Contact:
Brad Samson
[email protected]
M: 714.955.3951
Investor Contact:
Stewart Peabody
[email protected]
M: 312.498.5892
MKT-00010-27 Rev 1
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/kleiner-device-labs-kg2-surge-flow-thru-interbody-system-awarded-bronze-in-the-2022-medical-design-excellence-awards-301524744.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/kleiner-device-labs-kg2-surge-flow-thru-interbody-system-awarded-bronze-in-the-2022-medical-design-excellence-awards-301524744.html
SOURCE Kleiner Device Labs