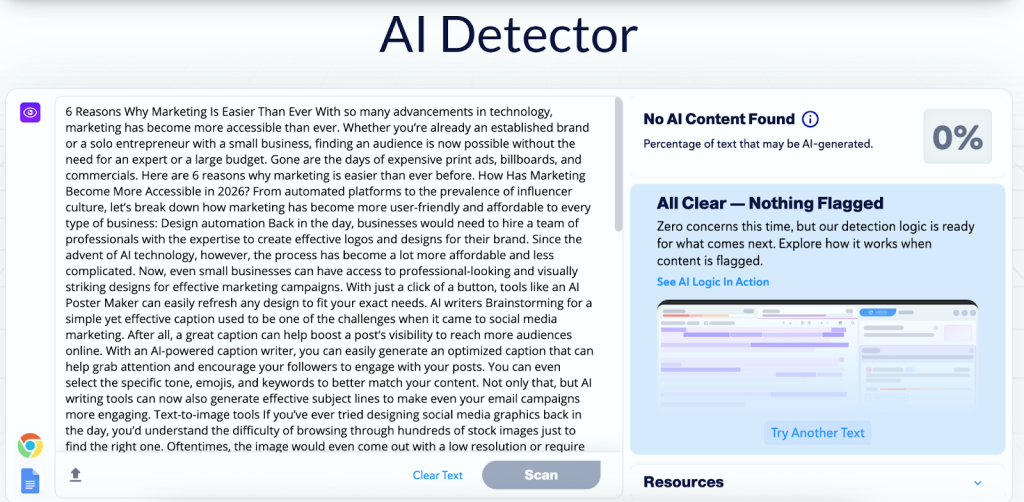
Samuel Ethiopia is CEO and co-founder of DocSpera, a digital healthcare company.
getty
As technology continues to improve and change all aspects of our lives, digital transformation is starting to emerge as a C-level objective in the medical device space. Data and tech enablement have become sources of competitive edge across product development and commercialization. It is changing how medical devices are delivered and deployed within the healthcare system to support cost-efficient, value-based models. In addition, technology and data enable robotic surgeries and drive efficiency across the supply chain, including just-in-time delivery of custom implants.
As speed to market is becoming critical to enable data and technology infrastructure, medical device leaders are racing to define and execute technology road maps and decide whether to build, buy or partner.
Below are three considerations for device leaders to establish strategic competitiveness.
1. Technology and data strategy should be a C-level decision and should have a singular oversight across all the product offerings
Medical device company leadership needs to establish a rigorous framework around its tech development process and a straightforward build, buy or partner strategy for its business development teams to adapt to ever-evolving technical requirements. Most medical device companies are still not equipped organically to pursue new data and technology opportunities. The iterative nature and pace of software development differ from the rigorous and regulatory-focused device development processes that have historically powered medical device companies.
Most medical device companies also typically lack the in-house expertise to build, commercialize and maintain cloud-based platforms and solutions. These activities require an understanding of data architecture, data security, systems infrastructure and agile development, which are non-core activities for medical device companies. With the proper senior leadership oversight, medical device players can ensure sufficient resources are required to acquire the right technology or digital expertise.
Ultimately, medical device companies will need to embed a data and technology strategy within their device and robotic navigation development process and include strategic decisions at all milestones during early-, mid- and late-stage development.
2. Building a focus on collaborating with agile startups is critical to driving innovations
Collaboration is essential to win in data and technology as many successful software capabilities are built on top of the open-source community. Contributions have catalyzed every digital health segment that has been able to scale from the significant incumbent healthcare players it seeks to disrupt. Payors have poured capital into digital health segments like care coordination and population health management that disrupt traditional fee-for-services models.
Similarly, hospital systems have dedicated resources to drive the growth and adoption of AI-driven clinical decision-making solutions, telehealth, remote patient monitoring and wellness solutions that ultimately reduce hospital utilization. Thus, medical devices need to understand, identify and work with startups building focused innovative technology solutions. Medical device companies can pilot and scale specific use cases faster by partnering with innovative technology startups while removing the risk of building resources, new software processes and capability from scratch.
Meanwhile, the startups benefit by scaling their solution through the well-established medical device sales channel and deployment processes. Thus, medical devices must establish a strategic partnership organization beyond traditional procurement that is adept at identifying innovation and able to scale the opportunity aligned to their corporate vision.
3. Having an operating model and vision that reflect a tech and data-enabled approach is essential
Once the vision and strategy are established to enable the company’s embedding of technology and data, it is essential to establish a clear organizational structure, process and capabilities. From an organizational structure perspective, having C-level leadership managing all digital initiatives across all business units will drive alignment and provide unified corporate KPIs to measure success. All the digital initiatives have to be embedded and prioritized within the business units to drive success.
As for the process, the organization needs to adopt an agile methodology to move at the same speed of technology development and scaling. In addition, identifying and onboarding technology partners needs to be outside procurement to accelerate and focus on the use cases relevant to the innovation ecosystem.
Finally, as for resources and capabilities, it will be essential to bring talents with technology and data experiences that can be a bridge between the technology need and medical device use cases. Holding frequent knowledge cycling and cross-learning cadences between the medical device, therapeutic and technology experts will allow the organization to grow and make technology and data part of its core DNA.
Evolving patient needs and provider requirements are the biggest drivers of changes in our healthcare system. Software and Technology have forever changed the lives of both of these user segments in every facet. With a well-articulated vision unique to each company’s goals, and real commitment, life science and medical device companies are uniquely positioned to lead our vast healthcare ecosystem into a more seamless and bright future.
Forbes Technology Council is an invitation-only community for world-class CIOs, CTOs and technology executives. Do I qualify?